News
News
PublISHED ON
UpDATED ON
260 ethically approved COVID-19 studies in Sweden so far
Since the pandemic begun this spring, 260 clinical studies relating to COVID-19 have been approved by the Swedish Ethical Review Authority. Getting results from most of these studies will take time, and for several studies it might be a challenge to find enough research subjects at the planned rate.
The Swedish Research Council summarises basic information about planned COVID-19 research in Sweden, based on excerpts from the Swedish Ethical Review Authority. The summary is updated and developed over time, and provides a current overview of the research planned. During April–June, almost 60 new studies per month were approved. Since the summer, numbers have fallen to approximately 20 approved applications per month.
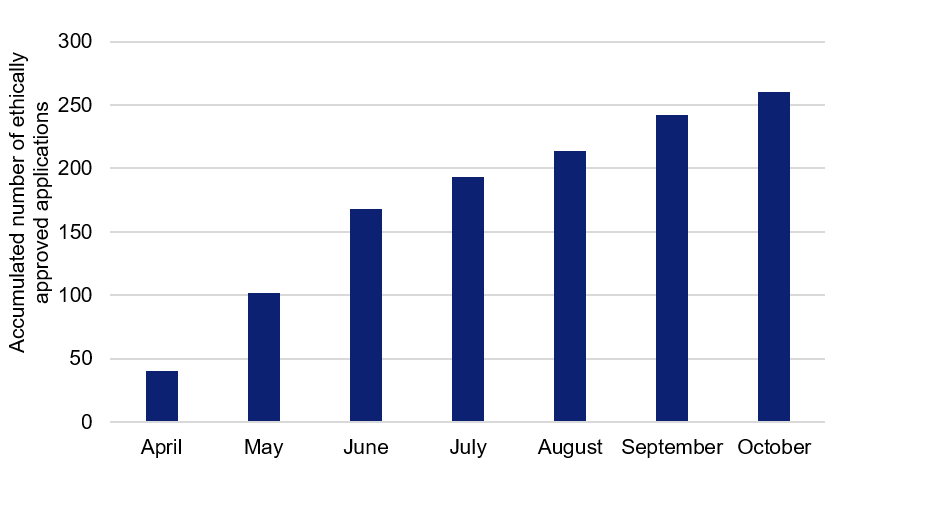
Figure 1. Applications relating to COVID-19, approved by the Swedish Ethical Review Authority during April–October (accumulated numbers).
“Researchers and research funding bodies have worked really quickly to contribute to research that can help to stop the spread of COVID-19 and preventing future pandemic outbreaks. Our summary now enables text searches in the project summaries. This makes it possible to find and thus avoid that similar research projects are being planned and funded,” says Madeleine Durbeej-Hjalt, the Swedish Research Council’s Secretary General for Medicine and Health.
Project period of six months or more for the majority of studies
The studies relate to many different kinds of research areas, from studying a small, specific patient group at an individual clinic, to major register-based studies analysing data covering Sweden’s entire population. 20 percent of the studies will be completed in six months or less, while more than half of them have a project period of one year or longer.
“In addition to the project period, time is often needed to summarise material and complete a report. For this reason, it will probably be some time before we see results from these studies,” says Madeleine Durbeej-Hjalt.
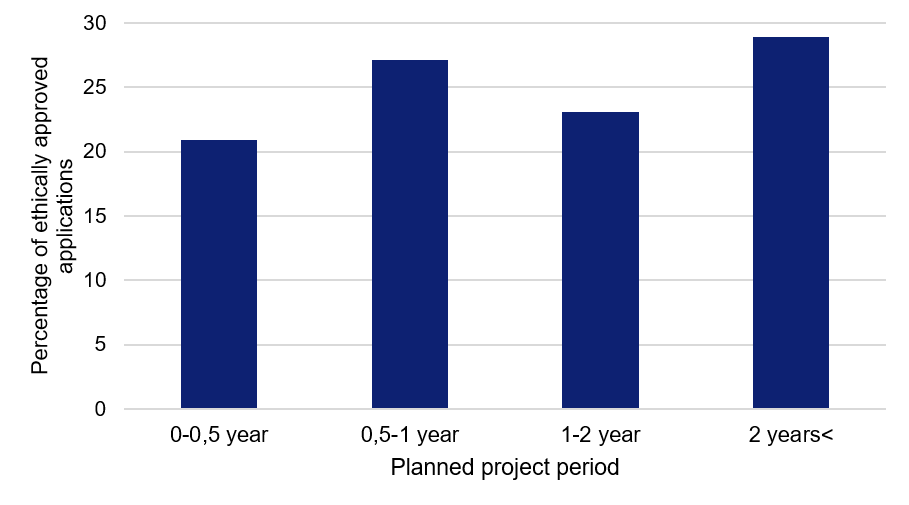
Figure 2. Planned project periods for the applications relating to COVID-19, approved by the Swedish Ethical Review Authority.
Several major studies with many participating research subjects
The applications approved to date show a large spread in terms of the planned number of research subjects. The median value is as large as 300 research subjects per study, and almost 80 percent of the studies plan to include 100 or more research subjects.
“Many large studies are being planned. At the same time, the patient population has changed considerably since the spring, which means that some studies may have difficulty finding and including enough research subjects at their planned rate. Few studies are conducted across several regions, and this reduces the flexibility of finding research subjects in the regions that currently have the highest number of patients,” says Madeleine Durbeej-Hjalt.
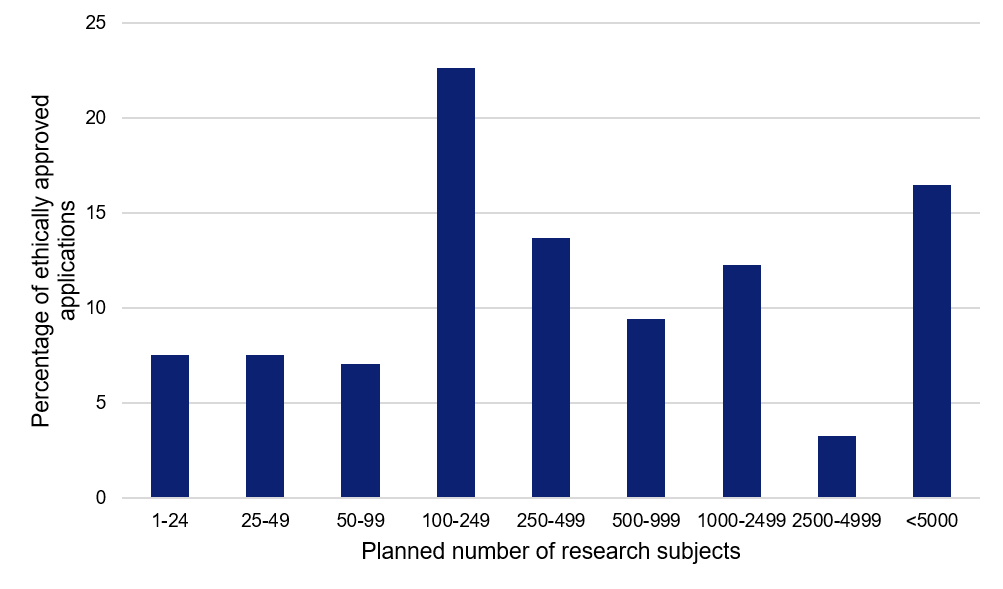
Figure 3. Planned number of research subjects for the applications relating to COVID-19, approved by the Swedish Ethical Review Authority.
High proportion of register-based studies
45 percent of the approved studies will be using register-based data. Two thirds of these only deal with personal data, and can be assumed to be pure register-based studies, while one third can be assumed to be studies including both patients and register-based data.
“Many of the larger studies, around 80 percent of those with more than 2 500 research subjects, plan to use register-based data, which explains the large number of planned research subjects,” says Madeleine Durbeej-Hjalt.
Brief facts about the studies
- 7 percent are medicine trials (this proportion is normally around 10 percent).
- 45 percent plan to use register-based data.
- 18 percent plan to include children.
- 7 percent are “multi-regional” (principals from several healthcare regions are taking part). Most of the studies by far are only conducted in, and using research subjects from, a single healthcare region.
Read the whole summary at kliniskastudier.se (in Swedish) External link, opens in new window.
External link, opens in new window.
MORE WITHIN THE SAME SUBJECT AREA
-
Activity |
Published 10 April 2024
On 12 of April, there will be an inauguration of one of the new centres of excellence with funding from the Swedish Research Council: Chemical Mechanisms of Life – an interdisciplinary centre for research into the chemistry of life. Mattias Marklund,...
Keywords:
-
Article |
Published 4 April 2024
The Swedish Research Council is funding 15 centres of excellence as from 2024. The centres, which are being built up, shall carry out ground-breaking research and educational activities around a central theme.
-
News |
Published 23 February 2024
As from 2022, all clinical studies funded by the Swedish Research Council shall be registered in a public database. A recent follow-up shows that the majority of the clinical studies awarded funding in 2022 have been registered.




