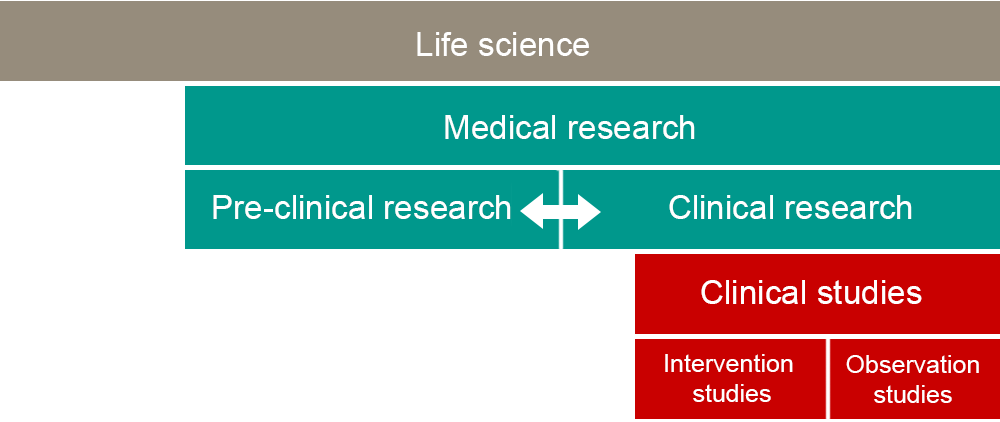
Concept structure
The structure illustrates how the concepts relate to each other – from the most overarching, life science, to the division of clinical studies into intervention and observational studies. The arrow between preclinical and clinical research symbolizes the mutual exchange between basic research and healthcare research infrastructures known as translational research. The idea is not for the boxes in the figure to correspond to the scope of research. The boundaries in the figure are not definitive either.
Life science
Life science is a broad concept, which can be described as the scientific field that studies living organisms. Knowledge in this field is usually developed through research in natural sciences as well as medicine and health sciences, but can also be multidisciplinary and include areas such as engineering sciences, ethics, social sciences and humanities.
One aspect of life science is solving the societal challenges that are linked to human health. For this purpose, collaboration between academia, health and medical care, the care sector, the business sector and relevant stakeholder organisations are of great importance. Examples of such issues are developing new and effective medicines, therapies, care methods, and new medical devices. This is often known as the “life science sector” in the business world, and in Sweden´s national life sciences strategy.
A national strategy for life science, government.se External link.
External link.
Medical research (research in medicine and health sciences)
Medical research provides knowledge of how the human body works and how disease can be prevented, how it arises, and is treated. Medical research aims to increase basic knowledge of how cells and tissues function and interact, and to contribute to the improvement and development of medicines, care and therapy methods, and medical devices.
Medical research is often divided into ‘pre-clinical research’ and ‘clinical research’, but both these interact, and mutual exchange takes place between basic research, the health of individuals (patients) and research infrastructures in healthcare. This is known as ‘translational research’ – that is, applied research aimed at bridging the gap between pre-clinical research and clinical research. The starting point for medical translational research is usually a problem identified in medical care, such as a need for treatment or diagnosis of a particular disease, and the goal is to speed up the application of research results in healthcare.
Pre-clinical research
Pre-clinical research is research in medicine and health sciences that does not require the structures and resources of healthcare. It covers basic experimental research at molecular, cellular and integrative level, relating to the basic processes of life that determine bodily functions – normally and during disease. The aim is to increase knowledge of how components such as cells, tissues and organs function and interact.
Clinical research
Clinical research is research in medicine and health sciences that requires access to the structures and resources of healthcare, and for which the aim is to solve a health problem, or identify factors that lead to improved health [ALF agreement, U2014/07551/F].
Clinical research is often conducted on human subjects, so called ‘clinical studies’, but there is also clinical research that is more similar to pre-clinical research. This applies, for example, to research that uses human material, and therefore needs healthcare resources, but where the purpose of the study is of a more basic character. Examples are studying and understanding how cells, tissues and organs function and interact, and how different disease conditions can arise.
All clinical research needs approval from the Swedish Ethical Review Authority.
Clinical studies
Clinical studies are carried out on human subjects in order to study medical or health-related questions. Clinical studies can, for example, relate to the study and/or development of medicines, therapy methods or medical devices, for the purpose of developing health and medical care. Examples of clinical studies are intervention studies and observation studies, including certain register-based studies. Clinical therapy research is covered by clinical studies and is therefore not included in the structure.
Intervention studies
In intervention studies, study participants are exposed to some kind of intervention, for example a medicine, a medical device, a diet, or a surgical method. Intervention studies that include medicines or medical devices are also known as clinical trials.
Observation studies
In observation studies, the study participants are observed without any influence from the researcher. Observation studies may consist of epidemiological investigations, such as cross-sectional studies, cohort studies, or case-control studies.
Register-based clinical studies
In register-based clinical studies, micro-data from national and regional quality registers or the registers of public agencies may be used to conduct epidemiological studies, or to plan, support or follow up intervention studies. Register-based clinical studies also include register-based randomised clinical trials (R-RCT) and biobank studies.
PublISHED ON
UpDATED ON