All clinical studies require ethical approval. The compilation below is based on data from the Swedish Ethical Review Authority’s annual reports, which contain aggregated information on the number of cases managed, and on data from an excerpt from the Authority’s register for the years 2020–2022.
The compilations show what type of analyses can be made on what data. To get increased understanding of the planned research and of individual studies, further information is needed, however. The Swedish Ethical Review Authority has an ongoing mandate to collect and present statistics for clinical studies in Sweden, and when supplementary and more detailed information becomes available, it is possible to carry out more in-depth analyses.
Ethical review applications received
The number of ethical review applications received relating to clinical studies usually falls within the range of 2 200–2 400 applications per year. From 2022, clinical trials on medicinal products managed according to the new EU regulation governing clinical trials on human medicinal products are reported as a separate category (CTR) in the annual reports.
The Swedish Ethical Review Authority’s annual reports also include information on the number of amendment applications. This category amounts to almost 3 000 per year, which is more than 50 per cent of the total number of cases. Amendment applications are not unique studies but relate to studies that have already begun and may, for example, be due to the researcher wishing to add more participating clinics (study sites) or collect additional data over and above what was stated in the original ethical approval.
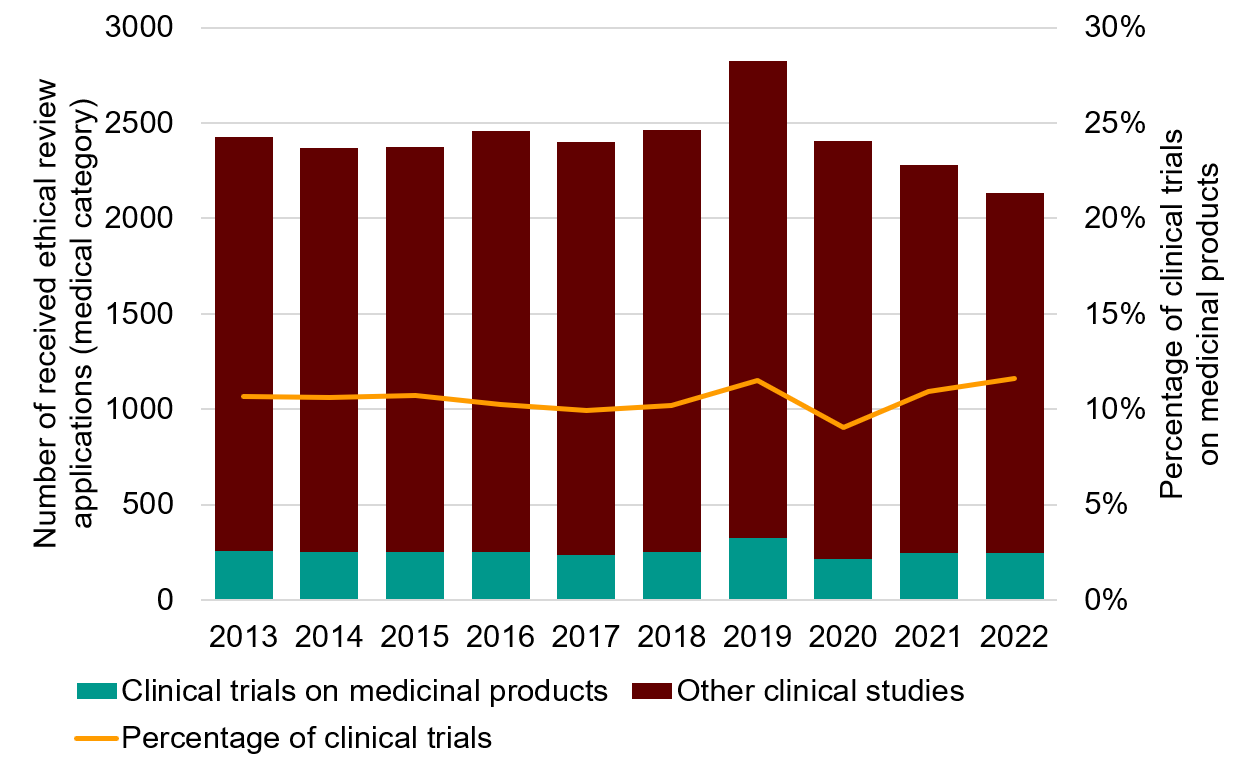
Figure 1. Ethical review applications received relating to medical research according to the annual reports for the regional ethical review boards (2013–2018) and the Swedish Ethical Review Authority (2019–2022), divided up into clinical trials on medicinal products and other medical applications (clinical studies). The increase in 2019 may be an effect of double counting of certain applications during the transition from the previous regional ethical review boards to the new Swedish Ethical Review Authority. For 2022, the applications have been included in the category “clinical trials on medicinal products” according to the new EU regulation (CTR).
Clinical trials on medicinal products | Other clinical studies | Percentage of clinical trials | |
|---|---|---|---|
2013 | 259 | 2170 | 11% |
2014 | 251 | 2117 | 11% |
2015 | 255 | 2120 | 11% |
2016 | 252 | 2205 | 10% |
2017 | 238 | 2160 | 10% |
2018 | 251 | 2210 | 10% |
2019 | 326 | 2501 | 12% |
2020 | 218 | 2190 | 9% |
2021 | 249 | 2030 | 11% |
2022 | 248,5 | 1887 | 12% |
Number of approved ethical review applications
A high proportion of all applications to the Swedish Ethical Review Authority are approved. During 2020–2022, 94 per cent of all applications relating to medical research were approved, which corresponds to around 2 000 applications per year. Two per cent were rejected, and the remaining four per cent consist mainly of applications that were retracted by the applicant or were refused because the application was not covered by ethical review legislation.
Nearly all research therefore receives approval to start, but fully covering statistics are lacking for how many of the studies actually do start, how many research subjects are included in the studies, and how many studies are completed.
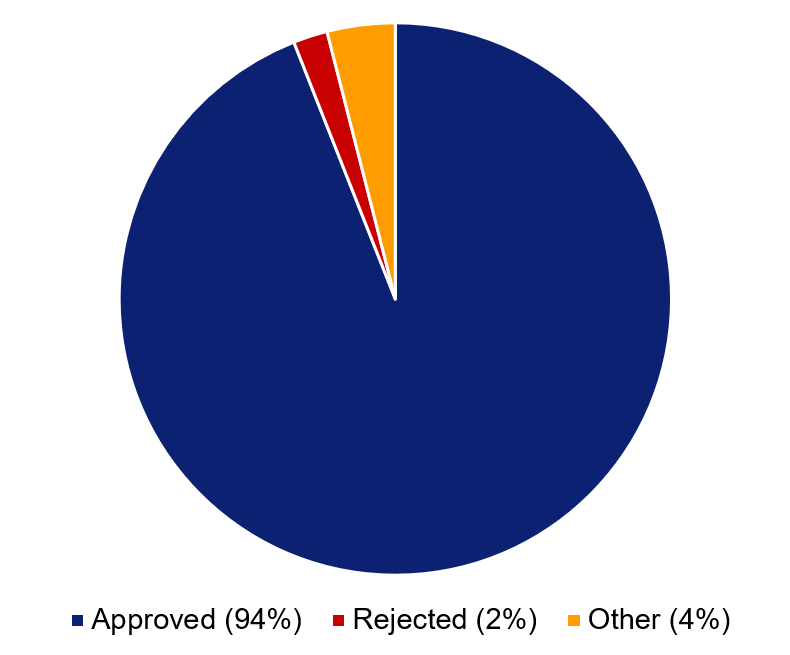
Figure 2. Distribution of decisions for ethical review applications during 2020–2022 relating to clinical studies (medical research). The category ‘other’ consists primarily of applications that were retracted by the applicant or were refused because the application was not covered by ethical review legislation. Amendment applications, or applications that resulted in advisory statements have been excluded.
Medical case type | |
|---|---|
Approved | 94% |
Rejected | 2% |
Other | 4% |
Geographical distribution of approved ethical review applications
The research principals in the Stockholm healthcare region are responsible for approximately one third of all ethical review applications approved. Thereafter follow the Mid-Sweden and Western healthcare regions, with around 18 and 17 per cent each respectively. In relation to the population size (according to population statistics from Statistics Sweden dated 31 December 2022) in each healthcare region, Stockholm has a higher proportion while the remaining healthcare regions have a smaller proportion in relation to their population shares.
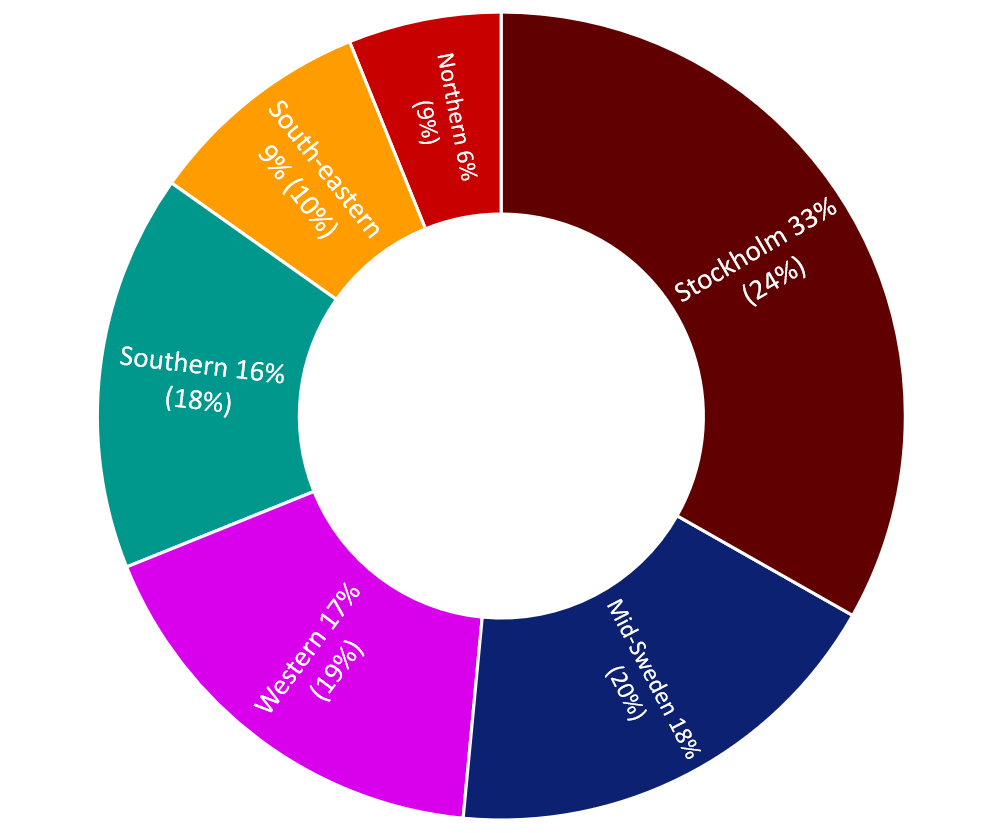
Figure 3. Geographical distribution (Swedish healthcare regions) of approved ethical review applications relating to clinical studies (medical research) during 2020-2022. The figure in brackets shows the population share for each healthcare region.
Application proportion | Population share | |
|---|---|---|
| Norhern | 6% | 9% |
Mid Sweden | 18% | 20% |
Stockholm | 33% | 24% |
Southeast | 9% | 10% |
Western | 17% | 19% |
South | 16% | 18% |
Distribution of ethical review applications based on fee category
The Swedish Ethical Review Authority’s fee categories give an indication of the type of studies that are planned. Clinical trials on medicinal products cover 11 per cent of the applications, and clinical trials on medical technology cover 1 per cent (the distribution is based on applications during 2020–2022). The category “Handling of personal data” covers 32 per cent of the applications and may largely be assumed to consist of studies that use data generated in healthcare. Other clinical studies are solely classed based on the number of principals particpating, and it is therefore not possible to make any more in-depth analysis of study types. Few studies have research subjects from several healthcare regions*. The most recent ALF evaluation(in Swedish) highlights that Swedish clinical research would benefit from more national collaborations, but that some regions in some research fields are currently competing rather than collaborating.
* It is in particular fee category B where it is clear that collaboration between the research principals is occuring. Supplementary information about participating principals is needed for all applications to enable any more in-depth analysis of the amount of collaboration for fee categories D and E, for example.
Fee category | Proportion of applications |
|---|---|
One research principal (A) | 43% |
Several research principals (B) | 7% |
Several research principals, but where the research subjects are linked to one research principal (C) | 7% |
Handling of personal data (D) | 32% |
Clinical trial on medicinal produts (E + CTR) | 11% |
Medical technology (MDR + IVDR) | 1% |
Proportion of approved applications relating to clinical studies (medical research) distributed across different application categories (fee categories) at the Swedish Ethical Review Authority during the period 2020–2022.
How the selection was done
Large parts of the data presented relating to ethical review applications are based on documentation with case information from the Swedish Ethical Review Authority’s register. When processing the documentation, the following selections and considerations have been made:
- Unless otherwise is stated in the figure texts, approved applications in fee categories A–E (relating to medical research), basic applications Part 1 for clinical trials on medicinal products (applications managed via the new regulation for clinical trials on medicinal products, CTR), and basic applications for medical technology have been included. This means that all types of amendment applications have been excluded, as well as basic applications Part 2 for clinical trials on medicinal products (to ensure the number of clinical trials on medicinal products is correct for 2022, as applications are submitted to the Swedish Ethical Review Authority in two parts and are managed as two separate cases). Applications resulting in advisory statements (around 70 per year) that are not covered by the definition of research in the ethical review legislation, and applications of case type “other”, have also been excluded.
- To link research principals to healthcare regions for private care providers and companies, the head offices or registered addresses have been used. These cases represent less than 5 per cent of the total number of applications. For some specific cases, it is unclear who the research principal responsible is, or the information is lacking in the documentation. These applications have been discounted in Figure 3.
PublISHED ON
UpDATED ON





