Approved COVID-19-related ethical review applications in relation to the transmission of infection
At the beginning of the pandemic in 2020, researchers started to plan new projects to study the disease, and, at the end of March, the first ethical review application was approved. Thereafter, the rate of new studies quickly increased and, during spring 2020, almost one in three ethical review applications related to COVID-19. During 2021, the rate of new applications gradually declined, and, during the last quarter of 2022, five applications were approved. If the three years are summarised, more than 500 applications were approved.
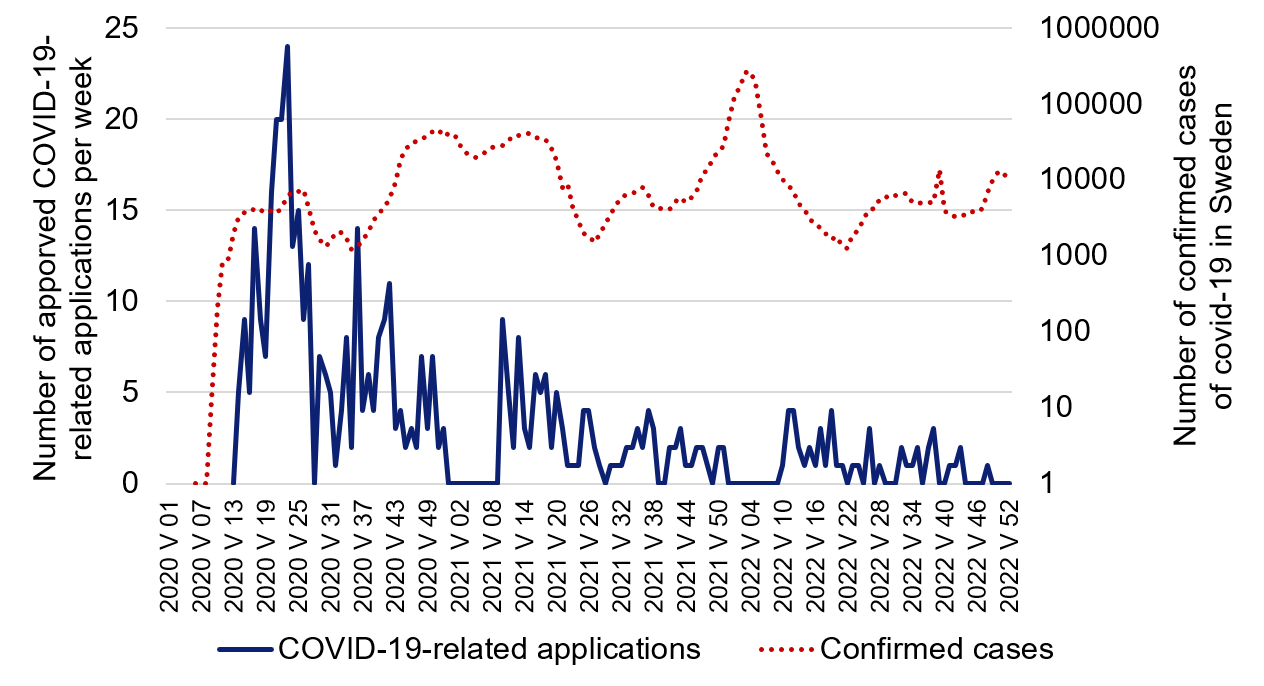
Figure 1. Number of approved COVID-19-related ethical review applications per week compared with the Public Health Agency of Sweden’s compilation (interactive fact sheet) of the number of confirmed cases of COVID-19 in Sweden on a logarithmic scale.
Report: Conditions for clinical studies during the COVID-19-pandemic
During the pandemic, the Swedish Research Council compiled information and diagrams based on the number of approved ethical review applications, to provide a picture of the clinical studies planned. The compilation showed that:
- register data were used in more than half the studies
- many research subjects were needed, as most of the studies planned to involve several hundred participants
- more than half the studies had a project period of one year or longer, which means that it might take many years before the research results are published
- few multi-regional studies were carried out. Most of the studies were conducted in and used research subjects from a single region.
Conditions for clinical studies during the COVID-19-pandemic (report in Swedish)
Approved COVID-19-related ethical review applications compared with other clinical studies
Researchers and healthcare personnel have reported that the work with other clinical studies had to take a back seat during the pandemic, while healthcare production and COVID-19-related research was prioritised (as shown in the report Conditions for clinical studies during the COVID-19-pandemic, in Swedish). At the same time, the total number of clinical studies remained stable during the years 2020–2022. This agrees well with the Swedish Ethical Review Authority’s assessment that the total number of applications received was not affected to any great extent (annual report 2020).
Throughout 2020, the proportion of COVID-19-related clincial studies amounted to 17 per cent of all applications, and during the following two years, the proportions were 8 per cent (2021) and 4 per cent (2022) respectively. During the period, the total number of approved applications was around 2 000 per year.
The rapid decline in COVID-19-related clinical studies was also confirmed by the Swedish Medical Products Agency, which in its annual reports (from the years 2020, 2021 and 2022) states that it dealt with 27 COVID-19-related applications for clinical trials of medicinal products in 2020, and 14 in 2021.
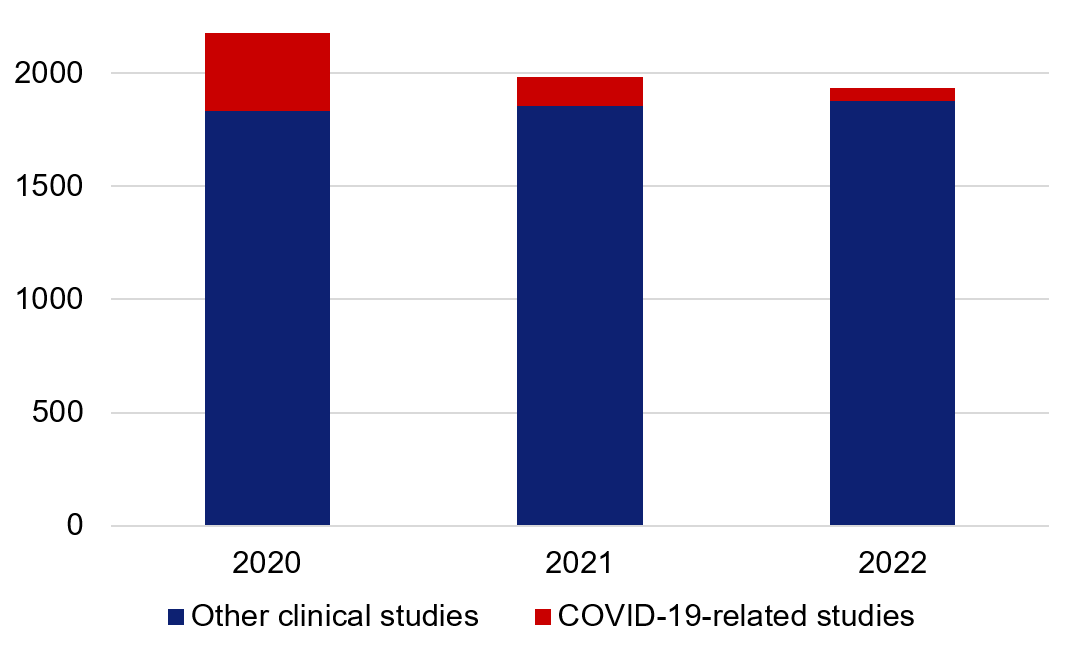
Figure 2. Total number of approved ethical review applications relating to medical research, divided up into COVID-19-related and other clinical studies.
Other medical applications | COVID-related applications | |
|---|---|---|
2020 | 1834 | 341 |
2021 | 1853 | 130 |
2022 | 1878 | 55 |
Proportion of approved COVID-19 applications
92 per cent of the COVID-19-related studies planned during the pandemic had their ethical review applications approved. 4 per cent were rejected (the remaining 4 per cent consist mainly of applications that were retracted by the applicant or were refused because the application was not covered by ethical review legislation). This is in line with other medical applications, where 94 per cent were approved and 2 per cent were rejected (average for 2020–2022).
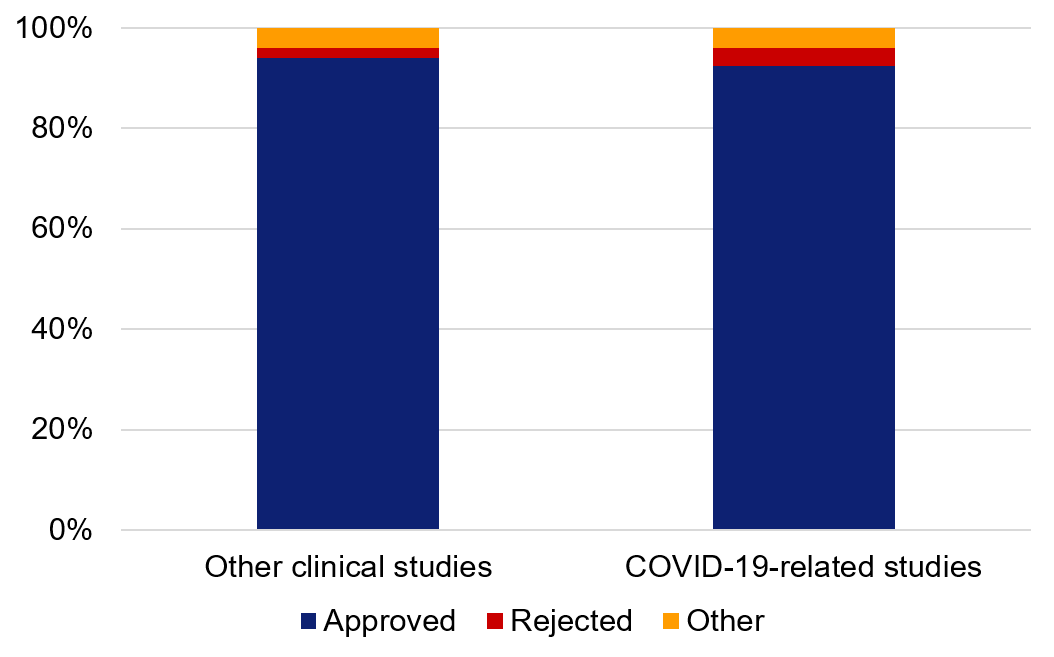
Figure 3. Distribution of decisions for ethical review applications during 2020–2022. The category ‘other’ consists primarily of applications that were retracted by the applicant or were refused because the application was not covered by ethical review legislation. Amendment applications, or applications that resulted in advisory statements have been excluded.
Other medical applications | COVID-related applications | |
|---|---|---|
Godkänd | 94% | 92% |
Avslag | 2% | 4% |
Annat | 4% | 4% |
Time until application
Early on during the pandemic, the Swedish Ethical Review Authority established a fast-track for COVID-19-related research, which may have caused some delay in the approval of other clinical studies (see table below). The effect was greatest during the second quarter of 2020, but by the end of the year the delay was around one week and continued thereafter at that level.
In 2022, it took around six weeks from submitting an ethical review application before a decision was made*. This includes administrative time for payment of fees and handling any supplements.
Year | COVID-19-related studies | Other clinical studies |
|---|---|---|
2020 | 37 | 58 |
2021 | 42 | 49 |
2022 | 40 | 45 |
The times include payment of fees and any supplements to the application*.
*The Swedish Ethical Review Authority states that the actual handling process was 35 days on average when based instead on the time from an application being both complete and paid for to the time a decision is made (according to the Swedish Ethical Review Authority’s annual report 2022).
Geographical distribution of research principals
During the pandemic, the distribution of COVID-19-related applications between healthcare regions was largely the same as for other clinical studies. The Stockholm, Southeastern and Mid-Sweden healthcare regions has a slightly higher proportion of the COVID-19-related applications compared with other parts of Sweden.
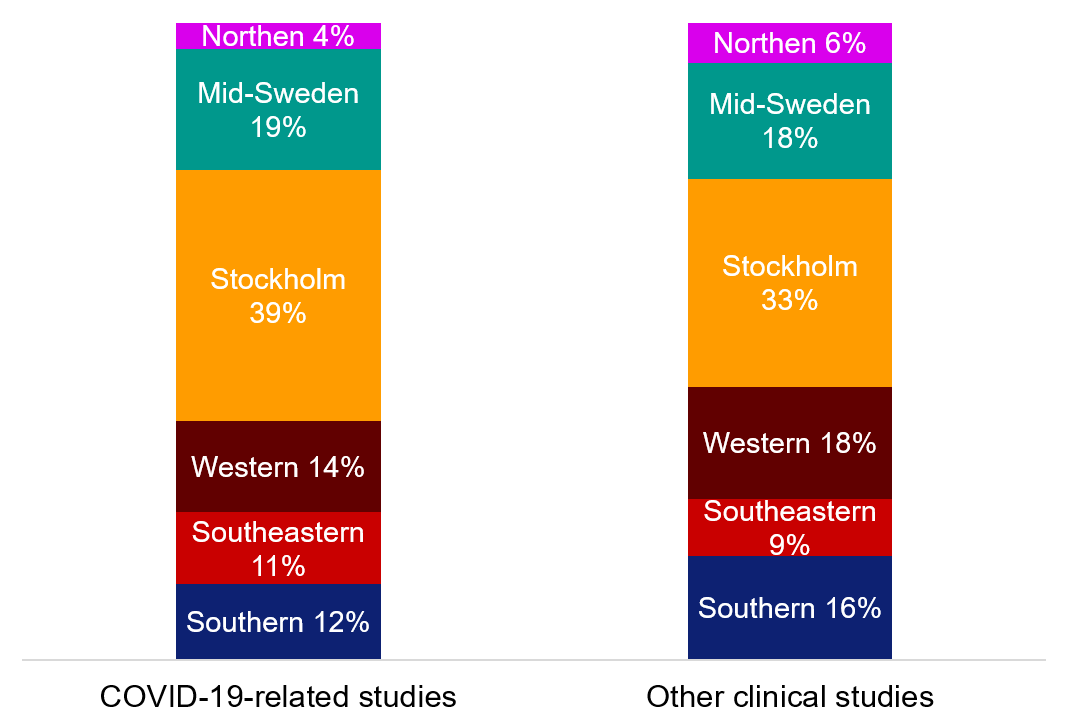
Figure 4. Geographical distribution of approved ethical review applications relating to clinical studies (medical research) during 2020–2022.
COVID-19-related clinical studies | Other clinical studies | |
|---|---|---|
Northern | 4% | 6% |
Mid-Sweden | 19% | 18% |
Stockholm | 39% | 33% |
Western | 14% | 18% |
Southeastern | 11% | 9% |
South | 12% | 16% |
PublISHED ON
UpDATED ON






